14+ calculate h3o
To do this can use the following. Web H 3 O HBr 075M Now we can calculate the pH of the solution with the equation pH -log H 3 O pH -log 075 125 Clearly this is a very acidic.
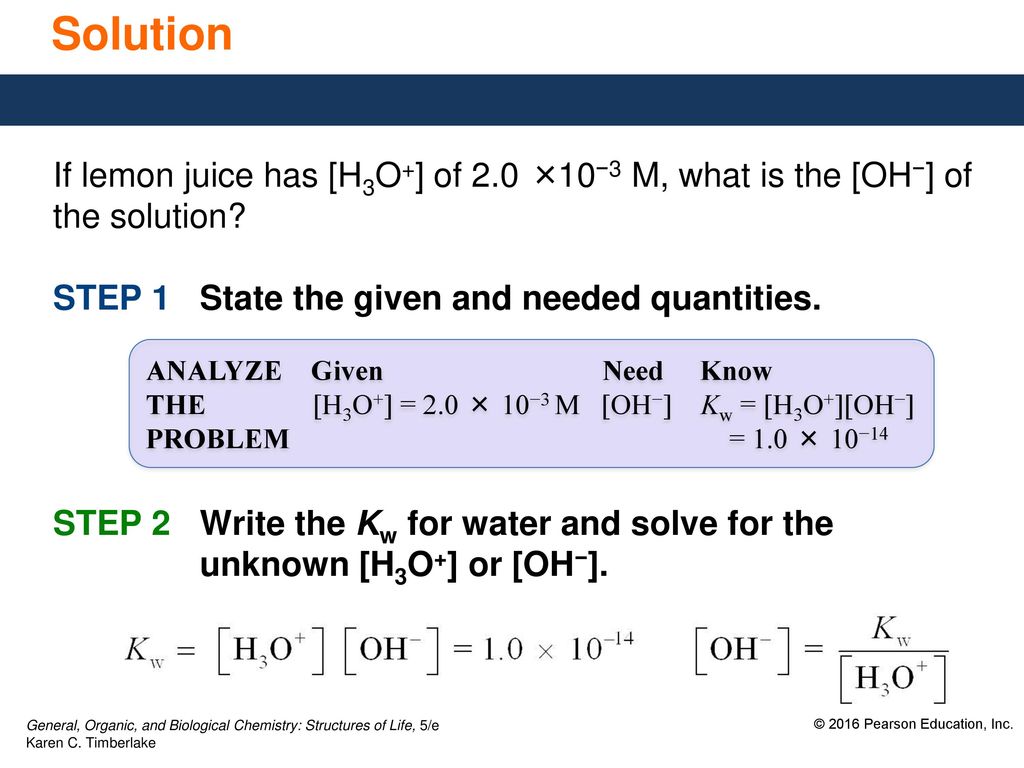
11 5 Dissociation Of Water The Equilibrium Reached Between The Conjugate Acid Base Pairs Of Water Produces Both H3o And Oh H2o L H2o L Ppt Download
In order to calculate pH we must take.
. Web The ionization constant for water kw is 9614x10-14 at 60 degrees C. Most often we use 1 x 10-14 as the Kw. Web Sorensen defined pH as the negative of the logarithm of the concentration of hydrogen ions.
OH- Ph and PoH for pure water at 60 degrees C. H2O l H2O l reversed arrows. Web In aqueous solutions H_3O is the strongest acid and OH is the strongest base that can exist in equilibrium with H_2O.
Web For example lets say a solution is formed at 25 degrees Celsius and the solution has a pOH of 475 and our goal is to calculate the concentration of hydronium ions in solution. Web How do you calculate H from pH. The leveling effect applies to.
While that might sound strange it does happen - water. H3O 10 -14 269 x. Web The ionization constant for water Kw is 29 1014 at 40 C.
In the following equation pH log H where H denotes the molar hydrogen ion concentration. Web If the concentration given is OH- or H3O use the relation OH- x H3O 10 -14 and solve for the unknown needed. Web Calculating the Hydronium Ion Concentration from pH The hydronium ion concentration can be found from the pH by the reverse of the mathematical operation employed to find the.
Since acids and bases react with each other this implies that water can react with itself. Web Autoionization of water. Web Its not a complicated question.
In terms of hydronium ion concentration the equation to determine the pH. Show more Show more 802 1417 The ionization. Calculate H3O OH pH and pOH for pure water at 40 C.
For example in A. 339 339 14 339 pOH 1061 As we have found the pOH we will now go ahead with finding the base concentration OH. Web The ionization constant for water Kw is 29 1014 at 40 C.
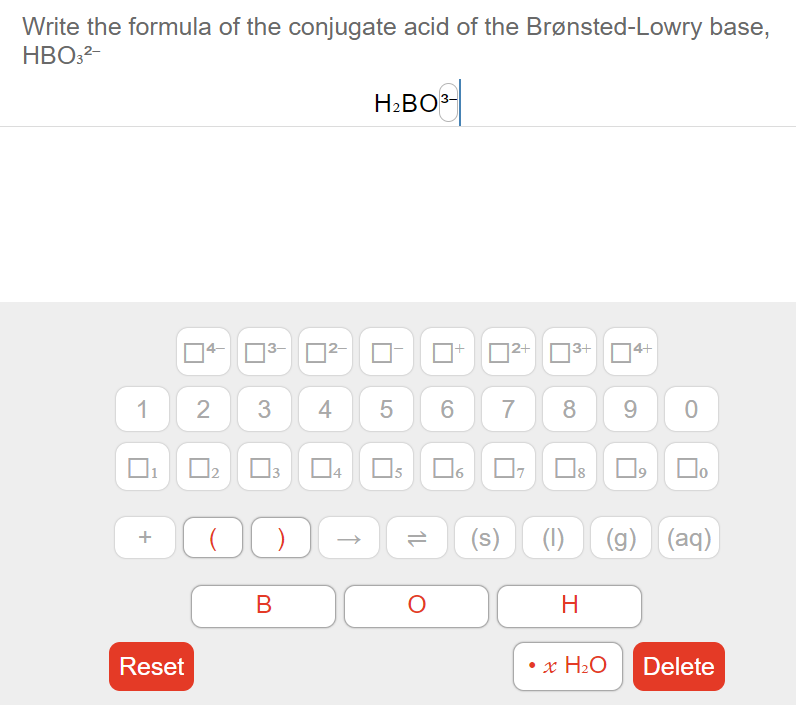
Web HSO3- The conjugate base of an acid always has one fewer proton and is one charge unit lower more negative than the acid Identify the Bronsted-Lowry acid the Bronsted-Lowry. Calculate H3O OH pH and pOH for pure water at 40 C. The product of H3O and OH- will always equal the Kw at the temperature specified.

Increasing Ubiquitin Ion Resistance To Unfolding In The Gas Phase Using Chloride Adduction Preserving More Native Like Conformations Despite Collisional Activation Analytical Chemistry

Electrically Conductive Metal Organic Frameworks Chemical Reviews
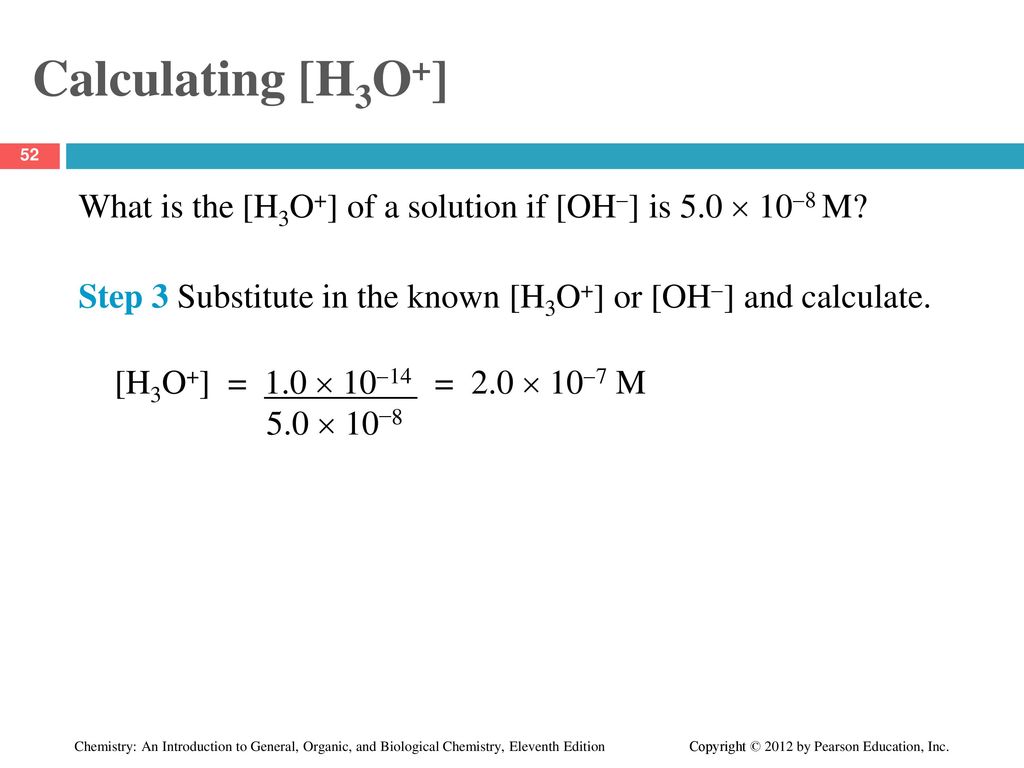
Chapter 8 Acids And Bases Ppt Download

Chapter 20 The Properties Of Acids And Bases

Calculate H3o And Oh Using Kw Mcmurry Ch14 Problem 55 Youtube
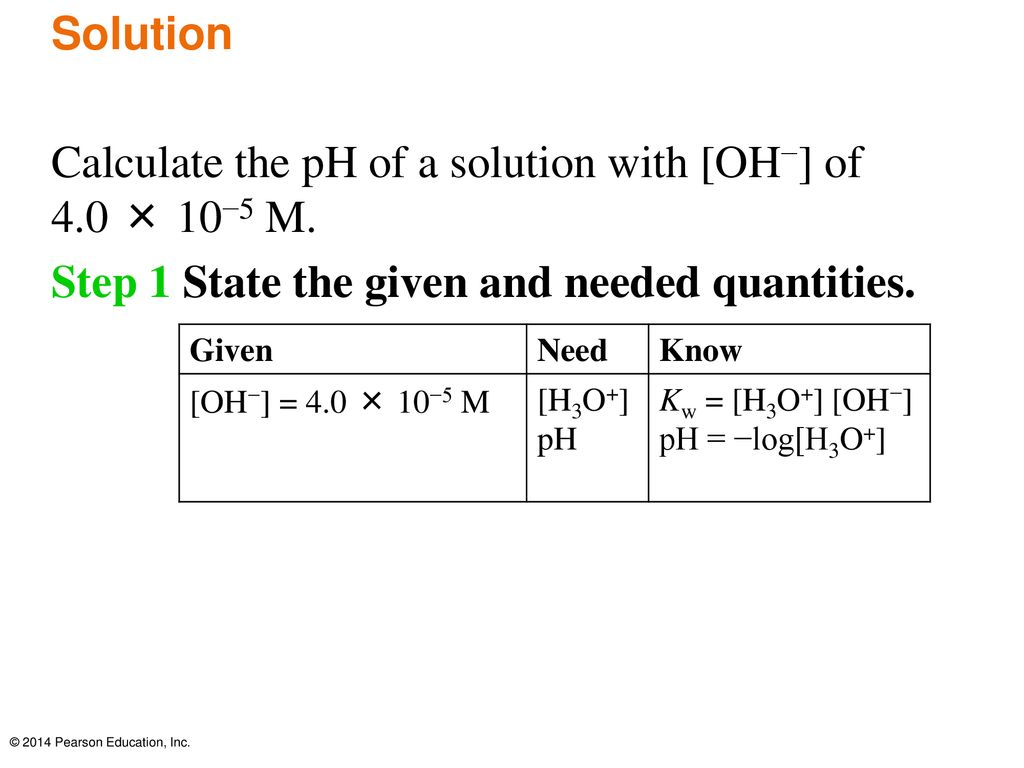
Chapter 14 Acids And Bases Ppt Download
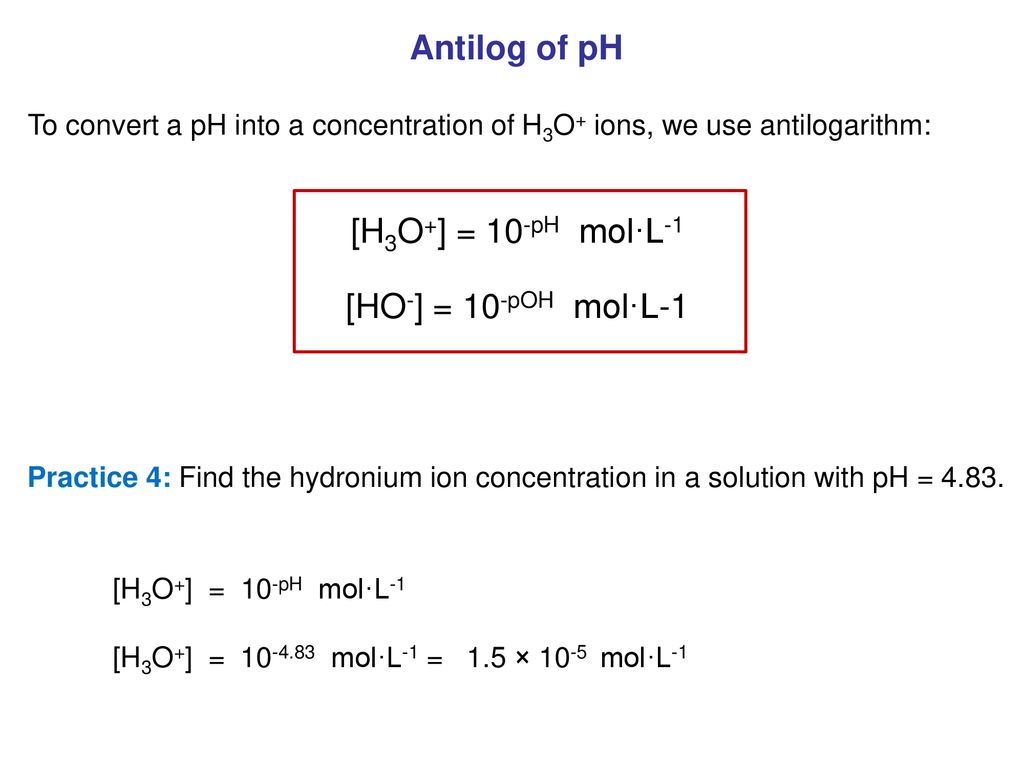
Chemistry 200 Focus 6 Acids And Bases Ppt Download

Chapter 16 Acids And Bases Ppt Download
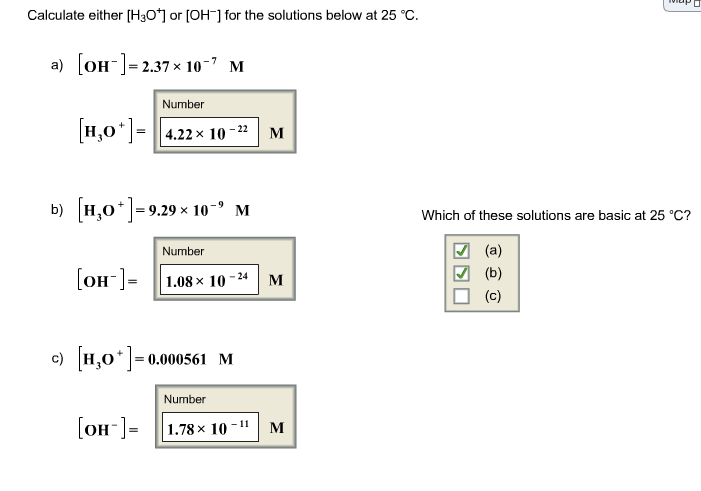
Solved Calculate Either H301 Or Oh For The Solutions Chegg Com

How To Calculate H3o And Oh Sciencing
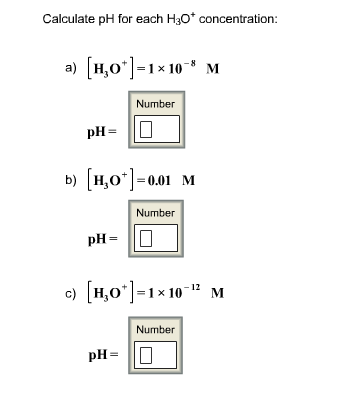
Solved Calculate Ph For Each H3o Concentration A H3o Chegg Com

How To Convert Ph To H3o Youtube

Acids And Bases Ppt Download
Solved Calculate Either H3o Or Oh For Each Of The Solutions Course Hero
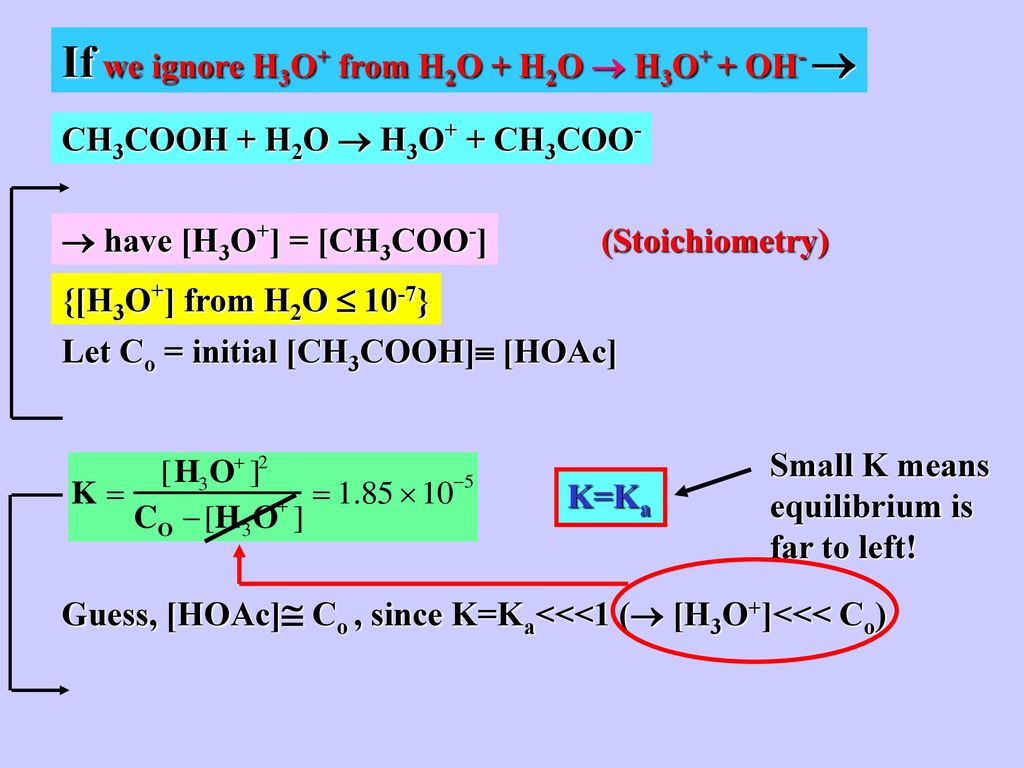
Suppose You Have H3o From Added Hcl 0 1 M 10 1 M Ppt Download
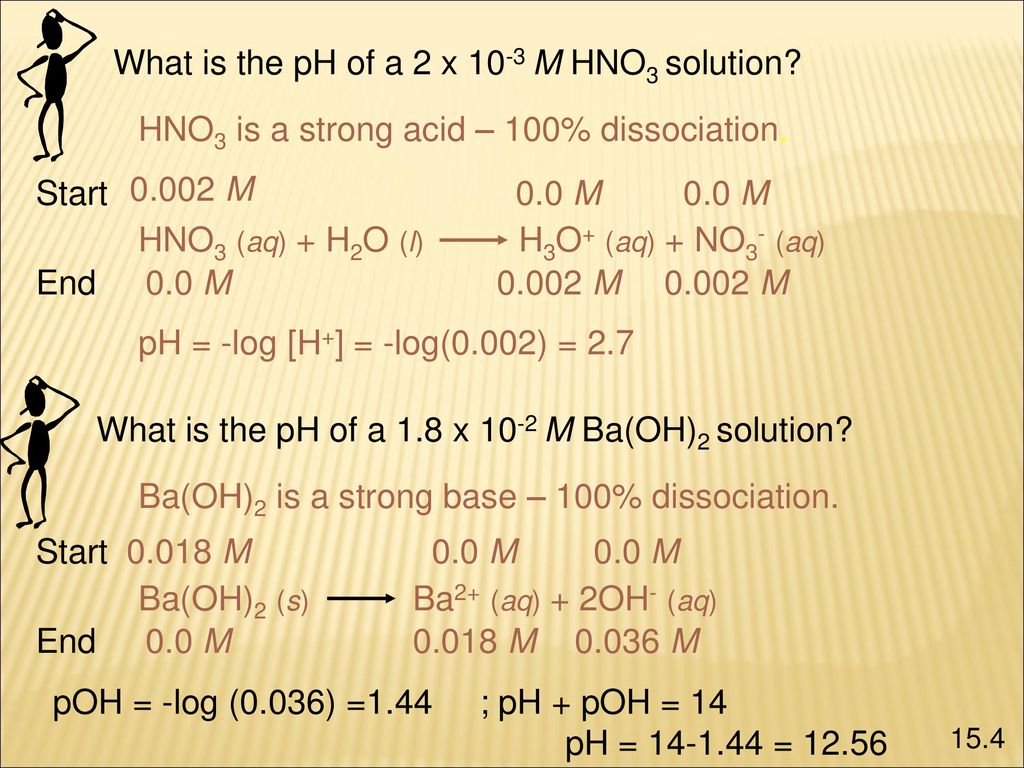
Acids And Bases Ppt Download

Calculating H3o From Ph Youtube